Analysis of the role of the pH in the anchoring of alkylphosphonic acid on a TiO2 surface
We are trilled to promote a new publication entitled “Analysis of the role of the pH in the anchoring of alkylphosphonic acid on a TiO2 surface: A DFTB study” prepared with the participation of our partner Institute for Organic Synthesis and Photoreactivity, National Research Council of Italy (ISOF-CNR) and Institute of Chemical Research of Catalonia (ICIQ).
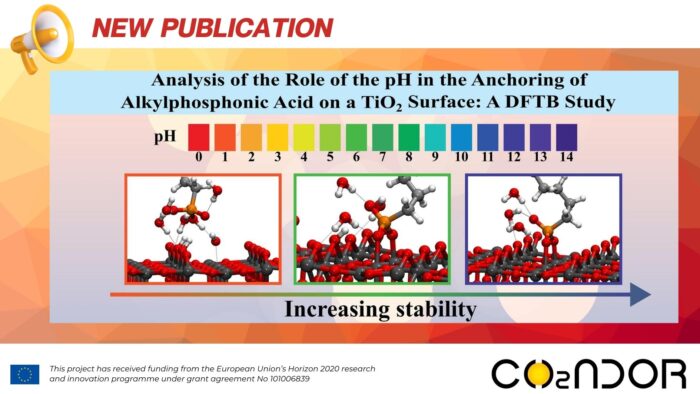
The reactivity of a (1 1 0) rutile titanium dioxide surface functionalized with neutral, anionic and di-anionic forms of dodecyl-phosphonic acid was studied by Density Functional based Tight Binding theory to simulate different pH conditions. Functionalization of this surface is relevant for at least two reasons: a) to protect the surface against external agents (e.g., by preventing the proliferation of bacteria in medical implants) and b) to use these organic–inorganic hybrid materials to facilitate the anchoring of other molecules. Comparing the results obtained in the gas phase and in water, experimental findings are better modelled by considering the hydration energy of the acids and the solvation-desolvation process involving the acids and the surface. In water, in all protonation states, acid molecules interact with the hydrated surface as a mono-negative charged species due to proton transfer before the grafting process. The formation of bi-dentate, di-anionic acid species, due to a proton transfer process or a change of pH, is favoured by anchoring alkylphosphonic acid to the rutile.
The publication can be accessible at the following link:
https://www.sciencedirect.com/science/article/abs/pii/S092702562200708X?via%3Dihub
.